Spectroscopy Notes
Electromagnetic Spectrum
The electromagnetic spectrum includes radio waves, microwaves, infrared light, visible light, ultraviolet light, x rays, and gamma rays. Visible light, which makes up only a tiny fraction of the electromagnetic spectrum, is the only electromagnetic radiation that humans can perceive with their eyes. (MS Encarta 1998)
Radio/TV and microwave waves are very big. They have a wavelength of several meters and even kilometers (and even bigger than that too!). Your eyes cannot see radio waves, but they enter and exit your house, your car, your body…etc at all times. Microwaves in your microwave oven are at a wavelength which will make water molecules vibrate so much that they heat up (causing your food to get hot. No water in the food would mean a microwave oven would be useless).
Visible light has smaller waves
than those of radio waves. Visible light has a wavelength of 400
to 700 nm. Visible light can be remembered by the acronym ROY-G-BIV.
Red has the longest wavelength at 700 nm and violet has the shortest at
400 nm.
Of course,
Visible light can be blocked by solid objects and it can be bent by liquids and
gases such as air and water. (look
at a pencil in a glass of water, it appears bent because the light going through
the water is slowed down by the water and makes the pencil appear “bent” to
your eye.) The sky is blue because
the molecules in our air bend the light from the sun so that blue light is
scattered and the other colors come shining through to our eyes.
The
sun may appear yellow or even red during certain times of the day, but this is
due to “color subtraction” from our atmosphere.
The sun itself is white if seen from outer space where there is no
atmosphere.
X-rays,
Gamma rays have wavelengths even smaller than those of visible light. They are down in the picometer range (and smaller than that
too!). These rays have harmful
effects on the human body. Our
atmosphere prevents many of these rays from reaching us (only light and radio
waves get through
What happens when you heat or
electrically excite a vaporized atom?
(eg. flame test of copper sulfate or electric current through neon
gas)
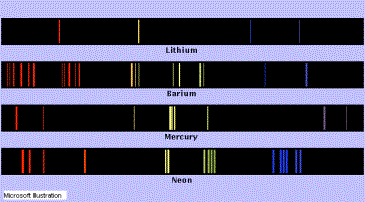
When a
substance is vaporized, and the vapor is heated until it emits light, a single
color may predominate, as in the yellow color of sodium-vapor lamps, the red
color of neon lamps, and the blue-green color of mercury-vapor lamps. The
spectrum in such cases consists of several lines of specific wavelength,
separated by regions of absolute darkness. In the case of sodium vapor, the
yellow color is produced by two lines of approximate wavelength 589.0 and 589.6
nm. The difference in color between these two lines is not detectable by the
human eye, but the lines may be readily resolved, or separated and
distinguished, by a good spectroscope. These two lines are called D2
and D1
(MS Encarta 1998) This
kind of spectrum is called an emission spectrum and can be seen below)

What
happens when you heat that atom to an exceptionally hot temperature?
(eg. The Sun or a
Light bulb)
The
simplest form of spectrum, called a continuous spectrum, is emitted by a solid
object that is heated to incandescence, or by a liquid or a very dense gas. Such
a spectrum contains no lines because light of all colors is present in it, and
the colors blend continuously into one another, forming a rainbow-like pattern.
A continuous spectrum can be analyzed only by spectrophotometric methods. In the
case of an ideal emitter, a blackbody, the intensities of the colors within the
spectrum depend only on the temperature. As the temperature is raised, the spectrum of blackbody radiation is
shifted toward the higher frequencies in direct proportion to the absolute
temperature.
(MS Encarta 1998). Cooler
blackbodies are red. Hotter are
blue. Try this experiment:
Use a dimmer switch on a light in your house.
Watch the color turn red-yellow-white
Absorption
spectrum of stars:
An interesting phenomenon, however, occurs when you look at the spectrum of the sun. Remember that the sun is white and therefore it emits all forms of electromagnetic radiation. This means that it emits (among other things) the complete visible spectrum from 400nm to 700nm (ROY-G-BIV). But, there are some “black lines” on the spectrum of the sun
(see
spectrum below).
These black
lines indicate elements present in the atmosphere of the sun which are absorbing
some of those wavelengths of radiation. Remember that Sodium emits light at 589.0 and 589.6 nm on the
electromagnetic spectrum? Well, it
also absorbs light at those wavelengths too.
You can see the two bands at “D”.
These bands represent the sodium that must be present in the Sun’s
atmosphere and are absorbing the light.
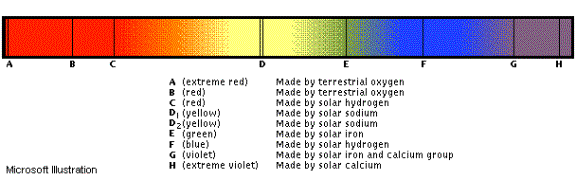
Spectrum of the Sun (above)
Spectroscopy
and Astronomy:
Astronomers
use spectroscopy and these “black lines” to tell what elements are present
in a star. Stars should give off a continuous
spectrum; but they don’t. The
black lines indicate which elements are present in their atmospheres.
On earth, we can take elements and heat them up with a flame and get
these “black lines” to come out as colored lines.
That is how we know which elements are in the stars.
In other words, if we heat up sodium on a Bunsen burner, we are going to
see two yellow lines in a prism. If
we take a spectrograph of the sun, those same two yellow lines are going to be
missing from the continuos spectrum. This
is because the sodium in the sun’s atmosphere absorbed those two yellow lines.
Cool
facts about stellar astronomy:
1. The element Helium was
discovered using spectroscopy. French
astronomer Pierre Janssen discovered helium in the spectrum of the corona of the
sun during an eclipse in 1868. Shortly afterward it was identified as an element
and named by the British chemist Sir Edward Frankland and the British astronomer Sir
Joseph Norman Lockyer.
The gas was first isolated from terrestrial sources in 1895 by the British
chemist Sir William
Ramsay, who discovered it
in cleveite, a uranium-bearing mineral. Natural
gas, which contains an average of 0.4 percent helium, is the major commercial
source of helium. The largest
producer of Helium in the world is Texas. (MS
Encarta 1998)
Between 1973 and 1980, the
natural gas companies were not required to recover helium from their pipelines
so they let it go (into the air and thus into outerspace).
Then, in 1980, scientists convinced the government to reestablish a
national helium reserve. Helium is used in a variety of places where an
non-combustible gas is necessary as a propellant or a cushion (eg. pushing hydrogen and oxygen fuel out of the space shuttle.
Cleaning air hoses for deep sea diving…etc). Despite these conservation
efforts, if present demands for helium continue the United States will exhaust
its helium-rich reserves by the year 2016
2.
If a star is moving away from the earth, the “black lines” on the
spectrograph move towards the red end of the spectrum.
This “red shift” or “Doppler effect” is how astronomers can tell
how fast a star is moving away from us or towards us.
A star moving towards us shifts toward the blue end of the spectrum.
As a star moves away, the wavelength of its emitted light is stretched.
Remember that red is the most stretched wavelength.
As a star moves closer, its emitted light is squished.
Remember that blue is the shortest wavelength.
(The Doppler effect can also be noticed by the sound of an approaching
train, siren or car and then be noticed by its change in pitch as it passes
you).
3. If a star is very hot, then the molecules of gas inside of it will be moving very fast. This makes those “black lines” of absorption spread out a little. Astronomers can tell the relative temperature of stars by looking at these spreading “black lines” of absorption. For example, the D1 and D2 yellow lines of sodium on our sun would be much broader on a star which is hotter than our sun.