Collection of H2 over H2O Lab
30 points
Click here for what is due for this lab
Purpose: To determine how many moles of hydrogen gas can be collected over water from the single replacement reaction of Magnesium and Hydrochloric Acid (HCl). And, to determine how many liters will hold one mole of this gas at STP (273K and 101.325 kPa)
Note: Anything in bold type means it is a line on the Data table which needs to be filled in.
Any number with a * in front of it needs to be put on a separate sheet of paper and show your work.
Procedure/Pre-lab questions:
*1. Obtain a piece of Mg ribbon and measure its mass in grams on a balance. Record this mass on your data table as Mass of Mg ribbon. (Mg reacts violently with HCl. Keep them apart at all times!). Figure out and record how many moles of Mg you have on your data table as Moles of Mg ribbon.
*2. Write out the
equation for this reaction. Magnesium
reacting with Hydrochloric Acid in a single replacement reaction. Record this on your data table as Balanced Equation.
3. Look at a thermometer and record the temperature of the room in Celsius and Kelvin on your data table as Temperature of the room.
4. Look at the barometer in the classroom and record the atmospheric pressure of the room on your data
table as Barometric Pressure. Remember our Barometer is in cm. You need to add a zero to make it mm.
And then turn it into kPas. (example: if it reads: “76 cm”, that means it is 760 mm)
*5. You will be using 10 mL of a 6 M solution of HCl. How many moles of HCl are in that 10 mL? Figure it out and show your work. Remember M = Molarity = moles/liter. Record this as moles of HCl on your data table.
*6. You can assume that you will have enough HCl to complete this reaction. Use stoichiometry and predict
how many moles of Hydrogen gas
will be produced. Record this on your
data table as Predicted moles of H2
produced.
*7. Now, take the predicted moles of hydrogen gas produced and use PV=nRT to predict how many mL of Hydrogen you should expect to collect given the amount of Mg you have for this experiment. Remember that V in PV=nRT is in Liters, NOT mL. Show your work and record this as Predicted Volume of Hydrogen Gas to be collected on your data table in mL.
----------------------------------------------End of pre-lab questions----------------------------------------------------
8. Show Mr. Young that you have completed procedure steps 1-7 on your data table and show him your
work on a separate paper and then you can go to the lab table and begin.
9. Take a long piece of Cu wire and make a loop in the end (as shown in this diagram). Wrap the Mg ribbon around the end of the loop.
![]()
10. Pour 10 mL of your 6 M solution of HCl into your gas collecting tube (that long skinny tube).
11. SLOWLY pour in the room temperature blue colored water on top of the HCl so that it fills the gas collecting tube all the way to the TIPPY TOP! The idea here is not to mix too much of the colored water with the HCl sitting at the bottom of the tube. You will see the food color near the HCl turn kind of green.
12. When the tube is
filled, it should look like this: Click Here For Figure A
13. Place your Cu wire with the Mg ribbon on it about 15-20 cm into the tube. Be careful not to let the Mg touch any of the “greenish” food color as this has acid in it. When the ribbon is in place, wrap the other end of your Cu wire around the open top of the gas collecting tube. Wrap it pretty tight. It should look like this when you are done:
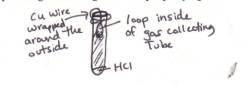
14. Now, place your finger over the hole in the gas collecting tube and turn it over into the large plastic graduated cylinder (filled to the tippy-top with water) provided at your table. The trick is not to let any air bubbles into the tube. If you do, then immediately record the volume of air you let in so that you can account for it later in the experiment. Click Here For Figure B which will show you what it should look like.
If you see a blank line, it means answer the question on the line
provided!
15. Because HCl has a molecular mass of 36.5 g/mole and water has a molecular mass of _______ g/mole, the HCl is more dense and will now begin to sink down the tube. As it does so, it will react with the Mg ribbon and start the reaction. What element is being released as a gas as this happens? _________
16. Once all of your Mg is gone, the reaction is complete. Which was the limiting reactant. The Mg or the HCl? _________________
17. Now that your Mg is gone, you need to determine the pressure inside of the gas collecting tube. Remember that if the level of the water in the gas collecting tube is equal to the water level in your large graduated cylinder of water, then the pressure inside the tube is equal to the atmospheric pressure of the room. If the water level in the gas collecting tube is HIGHER than the water level in the large graduated cylinder, then the pressure in the gas collecting tube is _______________(less or more – choose one) than the room pressure? Click here for figure C which will show you how to equalize the pressure in your gas collecting tube.
18. Read and record the volume of gas collected in your gas collecting tube as Volume of H2 gas collected on your data table
*19. Consult the
chart of vapor pressures of water at the temperature of the room. Use Dalton’s law of partial pressures to
subtract the water pressure from the hydrogen gas pressure. To do this, subtract the kPa value of water
vapor pressure at room temperature from the kPa value you got for Barometric Pressure of the room. Realize that the pressure inside the gas
collection tube is a combination of the hydrogen gas as well as the water
vapor. You are subtracting out the
water vapor in an effort to determine the pressure of just the Hydrogen
gas. Now, record the answer you get on
your data table as Pressure of H2
gas collected.
*20. So now you know
the Pressure of the H2 gas
collected (P1). You know
the Volume of H2 gas
collected (V1). You know
the Temperature of the room (T1)
and you know R = 8.315 kpa(L)/mol(K).
Now, you need to solve for the number of moles of H2 produced
using PV=nRT. Record your answer as Moles of H2 produced on your
data table. (and show your work for this on your paper). Remember, your gas
collection tube records volume in mL.
But if you use PV = nRT, you need to have volume in units of Liters. If you don’t make this conversion first, the
number of moles of H2 produced you’ll get will be incorrect.
21. Okay, so now you know how many moles of H2 were produced. How far off were you from your Predicted moles of H2 produced? Subtract your predicted value from your actual value of moles produced and put that answer on this line ________________.
22. Okay, so now you also know how many mL of hydrogen gas you collected (Volume of H2 gas collected). How does this compare to Predicted Volume of Hydrogen Gas to be collected? Subtract the two to see how close you were and put the answer on this line __________________.
*23. Now you know the number of moles of hydrogen produced. Take this number and put it into a PV=nRT equation so that you have STP conditions (eg. P = 101.325 kPa and T = 273k). And solve for the Volume. Record this answer on your data table as Volume of H2 at STP. This represents the volume your collected hydrogen gas would occupy at STP conditions.
*24. Finally, you can determine the volume of 1 mole of hydrogen gas at STP by using the following equality: (1 mole of H2 means just that ONE – as in the number one)
moles of H2 produced = 1 mole of H2
Volume of H2 at STP x where x = volume
of 1 mole H2 at STP
Put your answer on your data table.
Record volume of 1 mole H2 at STP on your data table.
Data Table:
BEFORE EXPERIMENT
DATA:
Mass of Mg ribbon__________g
Moles of Mg ribbon__________mole
Balanced Equation____________________________________________
Temperature of the Room___________oC _______________K
Barometric Pressure__________mm of Hg _____________kPa
Moles of HCl____________mole
Predicted moles of H2 produced _____________mole
Predicted Volume of Hydrogen Gas to be collected _____________mL
AFTER EXPERIMENT DATA:
Volume of H2 gas collected
____________ mL
Pressure of H2 gas collected ____________kPa
Moles of H2 produced _______________mole
Volume of H2 at STP ______________mL
volume of 1 mole H2 at STP
______________mL
Post Lab Questions:
*1. Use your knowledge of stoichiometry and limiting reagents to determine the number of moles of HCl which will be left over after the experiment.
*2. Convert these moles from Question #1 into grams and tell me how many extra grams you had of HCl.
*3. Did your volume of 1 mole H2 at STP value come out close to Avogadro’s prediction of 22,400 mL?
Figure A

Figure
B
Notice that the graduated cylinder is filled to the tippy-top with
water

Figure
C Notice that the level of the water in the gas collecting tube
is equal to the water level in the plastic graduated cylinder.

1. Answer
questions 1, 2, 5, 6 and 7 from the Procedure/Pre-lab questions:
(you don’t need to copy the questions – just number them 1, 2, 5, 6 and 7) Make sure you show your work!
2. Answer questions 15, 16, 17 and 19-24 from the procedure. Remember that any question with an asterisk (*) next to it requires some work shown. Make sure you show your work.
3. Data Table
4. Post-Lab questions (again, be sure you show your work)
Neatness counts. Please make sure that you use the same numbering I used in the lab (eg. Question 1, 2, 5, 6, 7…etc)