Chapter
10 – Gases
1. Volume, Temperature, Pressure:
Volume can be expressed as: L, dm3, m3, mL or cm3.
1L = 1dm3 and 1mL =
Temperature
can be expressed as: oC,
oF or K (Kelvin) (p.
317)
Kelvin = 273 + oC
oC = Kelvin – 273
Temperature is a measure of the Kinetic Energy of a gas. The
higher the temperature, the higher the KE.
You need to know the temperatures of Boiling water, freezing water, dry
ice solid, your normal body temperature and liquid nitrogen’s boiling point in
all three units of temperature. KE
= 1/2 mass (velocity)2. Large
gas particles move slower than small.
Pressure
can be expressed as kPa, atm, mm Hg, torr, bar, Pa (pascals) and psi (pounds per
square inch). Pressure is a measure
of the force exerted by a gas over a given area.
(Remember that area is a two dimensional space – not 3 like VOLUME.)
You need to memorize
these standard air pressures at sea level:
101.325
kPa
10058.4 mm of H2O
1
atm
760
mm of Hg
396 inches of H2O
14.7
psi
29.9 inches of Hg 760 torr

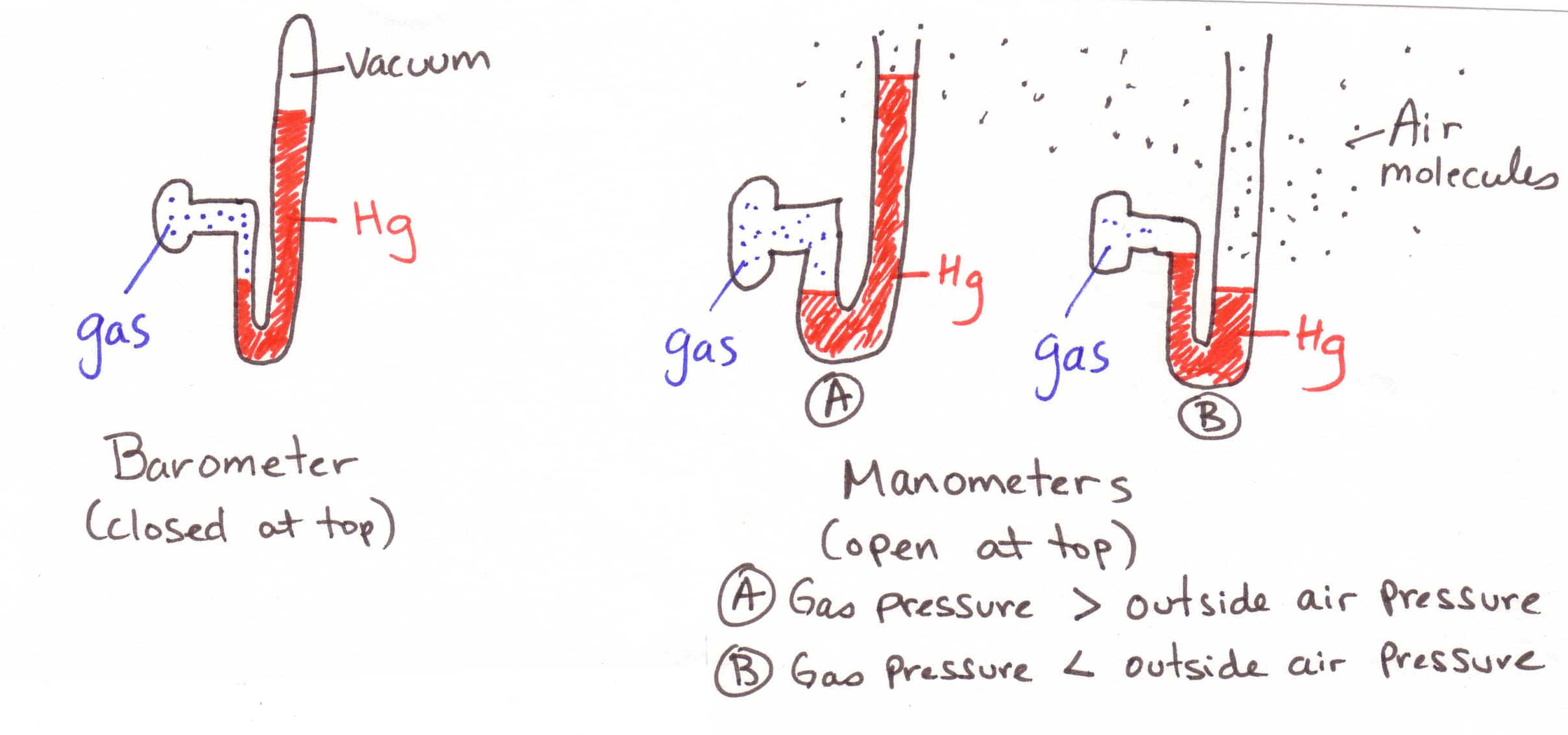
2. Barometers/Manometers
(See drawings above)
Know the difference between the two and how to work problems associated
with each. I suggest that you read
the chapter on this (p. 310 – 311)
3. Dalton’s law of Partial pressure: (p. 322 – 325)
Each gas gives off a pressure based on the number of molecules, the
temperature of the gas, and the volume in which is contained.
It is IMPORTANT to know that no matter what the gas is (hydrogen,
nitrogen, water vapor…whatever), the pressure is based on these three things:
Number of molecules, Temperature, and Volume. NOT DEPENDENT ON THE MASS OF THE GAS MOLECULES THEMSELVES!
Dalton’s
law states that if you put two gasses together, their total pressure will be the
sum of their individual pressures. This
assumes you are adding equal VOLUMES of gas to an equal VOLUME.
(eg. one liter of oxygen plus one liter of hydrogen being
combined into a one liter container).
How
to do problems involving Dalton’s Law:
1. When you collect a gas over water it is very IMPORTANT to know that when the water level inside the gas collection container is equal to the water level in the bucket, the gas pressure above the water is equal to the outside air pressure. If the water level in the gas collection container is BELOW the water level, then the pressure in the gas collection container is GREATER then outside air pressure. If the water level is in the gas collection container is ABOVE the water level, the pressure is LESS. (See drawing below)
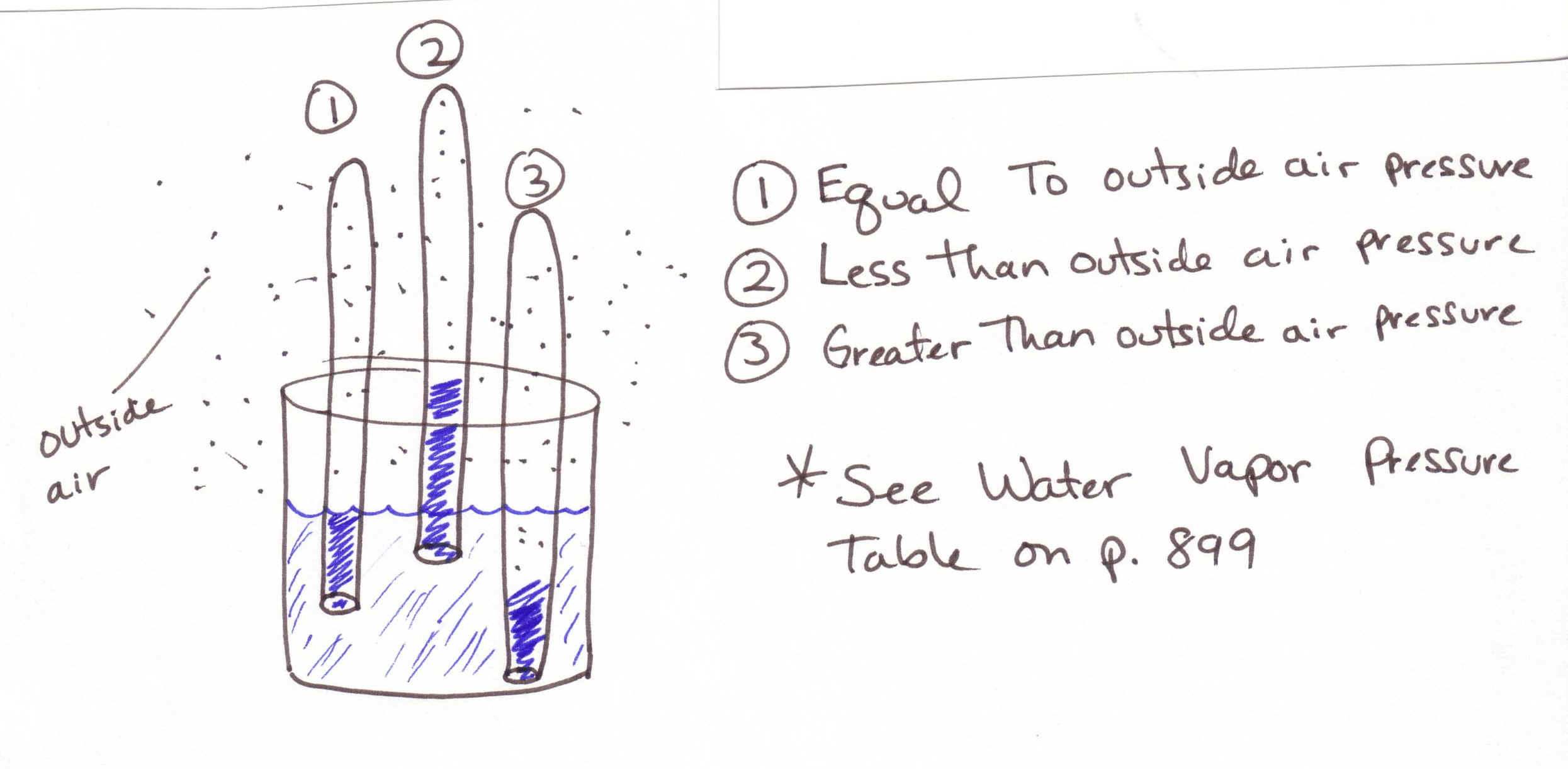
2. Vapor
pressure is the tendency for a liquid to turn into a gas.
Vapor pressure of water is dependent upon the temperature of the water.
Hotter water has a higher vapor pressure. The greater the vapor pressure,
the greater amount of liquid is turning into a gas.
(recall that water has a low vapor pressure – lower than say gasoline-
because of strong intermolecular forces, but that water’s vapor pressure will
increase when it is heated. Any
liquid’s vapor pressure will increase when it is heated).
See Table A-8 p. 899
3. Dalton’s law states
that the pressure inside of your collection container will be created by the sum
of the pressures of: The water
vapor pressure and the pressure of the gas you have collected.
To solve a problem, simply find water’s vapor pressure for the temperature of water you have in your container and subtract that from the atmospheric pressure of the room. That will give you the pressure of the gas you collected in the container.
Fascinating Fact:
Gases (such as He, N2, H2…) have low
intermolecular forces and thus have high vapor pressures and low boiling points.
The more massive gases have stronger I.F.s so as you go left to right on
the periodic table, the boiling point of gases increases.
Except He has a lower b.p. than H2.
Reason: H2 has a
stronger dispersion force (see Ch. 6 notes) created because H needs one more
electron to be a complete outer level. But
He is a noble gas.
b.ps. of some gases: He =
4K, H2 = 20 K,
Ne = 27K, N2 = 77
K, O2 = 90 K, F2
= 85 K
4. Charles’ Law and Boyle’s Law:
ALL TEMPS ARE IN KELVIN – NO EXCEPTIONS!
Charles’ Law: At
a constant pressure and a constant
number of molecules, the volume of a gas will increase proportionally
with the increased temperature of the gas.
Memorize:
V2T1 = V1T2.
Boyle’s Law:
At a constant temperature and a constant
number of molecules, the volume of a gas will decrease proportionally
with the increased pressure on the gas.
Memorize:
P1V1 = P2V2.
Pressure/Temperature Law:
At a constant volume and a constant
number of molecules, the pressure of a gas will increase with an
increased temperature on the gas.
Memorize:
P2T1 = P1T2.
(Gay-Lussac’s Law)
Fascinating Fact:
Liquid Nitrogen is made by increasing the pressure on nitrogen gas (to
about 4 atm) while keeping the volume constant.
This makes the nitrogen molecules come together and intermolecular forces
bind them together as a liquid. While
this is being done, the temperature increases dramatically (to about 1150 K). Liquid nitrogen is then stored in giant pressure tanks which
radiate the heat into the surrounding air until the liquid cools to 298 K (room
temp). When the liquid is released,
it is exposed to regular atmospheric pressure (about 1 atm) and the temperature
of the liquid drops dramatically to 77 K with the drop in pressure.
5.
Avogadro and Gas Laws: (This is
actually described in Chapter 11 p. 333-337)
Amadeo Avogadro (the guy who created the mole concept) said that equal
volumes of gases (at equal temperatures and pressures) contain equal number of
particles. This means that 1 liter
of water vapor has the same number of molecules as 1 liter of nitrogen gas.
Even though the Nitrogen gas would be heavier/larger, it still would have
the same number of particles. (Fascinating fact: Humid
air is lighter than Dry air. WHY?
Because dry air is mostly N2 and O2 with an average
mass of about 30 g/mole. Humid air
has water in it and water is only
18
g/mole. Therefore, an equal volume
of humid air will have a mass of less than 30 g/mole! What does this mean? It
means that when the barometric pressure is low, we have a damp day – and a
storm may be moving in. When the
barometric pressure is high, we have a dry day and clouds may be on their way
out).
Think
of Avogadro’s principle like chairs in a room.
If there are 30 chairs, they can be filled with 30 people.
This can be 30 small people, 30 large people, or a mixture of 30 people.
But the limit is 30!
Avogadro said that at a Standard Temperature and Pressure (STP)
of 1 atm and 0oC, that
one mole of any gas would occupy a volume of 22.4L
Important fact: Volume of a gas is dependent on three things:
1. Pressure on the gas
(inversely related)
2. Temperature of the gas
(directly related)
3. Number of particles in
the gas (directly related)
Also: Liquids boiled into a gas will occupy a larger volume as a
gas than as a liquid. All liquids
occupy less volume when frozen solid – except water, which expands when it is
frozen.