Barking
Dog Lab
20 Points
Purpose:
To use your knowledge of writing and balancing equations to predict the
products of two reactions.
Materials:
Ringstand
Pneumatic Trough
Rubber Stopper
Erlenmeyer Flask
Test Tubes
Glass tubing
Aluminum Foil
Matches
6.0 M HCl
Procedure:
- Obtain
an Erlenmeyer flask attached to a ring stand with a stopper and tube
attachment. Also obtain a small
piece of Aluminum Foil.
- Put
your SAFETY GOGGLES ON! DO
NOT TAKE THEM OFF DURING THE ENTIRE LAB! OTHER GROUPS WILL STILL BE WORKING!
- Measure
out 20.0 mL of
6.0 M HCl and pour it into the flask. BE
VERY CAREFUL AS ACID WILL BURN SKIN. IF YOU GET ANY ACID ON YOU, BE SURE TO RINSE IMEDIATELY
WITH WATER AND PUT BAKING SODA ON THE AFFECTED AREA.
- Fill
your test tubes HALF-WAY with water
and set them up in the Pneumatic Trough so that they are upside
down.
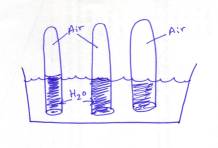
- When
you are ready, crumple the Al foil into a light ball and put it in the
Erlenmeyer flask. PUT
THE STOPPER ON FIRMLY! Put the hose UNDER THE
WATER when you put the stopper in place The pressure inside the flask
will build and you donít want the stopper to pop off!
(If it does pop off, donít panic!
Just be sure to rinse off any acid that might get on you and call me
over immediately! If the rubber
stopper pops off, it rarely splashes acid, but please be careful and be
prepared.)
- After
a few seconds, the reaction will take place. It happens RAPIDLY so work quickly.
Remember: Put the hose UNDER THE WATER when you put the stopper in
place. When the reaction begins, collect the bubbles under the test
tubes. When the bubbles are all
collected, lift the test tubes (one at a time) out of the Pneumatic Trough
and light a match underneath the mouth of the test tube.
Donít turn the test tubes over (so their mouths are pointing up) or
else youíll lose all the Hydrogen and have nothing to light. DONíT
LIGHT THE HOSE COMING FROM THE ERLENMEYER!
- Throw
your matches in the pneumatic trough BEFORE putting them in the trash can.
We donít want any trash can fires!
- Woof!
Pre-Lab Questions:
Answer these before doing the lab.
Bring them to class with you. No
need to copy the questions
- Should
the Aluminum foil be crumpled up tight, loose or left as a flat sheet?
- Should
you put the stopper on firmly or lightly?
- How
many mL of acid are you going to add to the Erlenmeyer flask?
- How
much water should you put in the test tube?
- Where
should you put the hose from the Erlenmeyer immediately after you put the
stopper in place?
- How
should you hold the test tubes so that you can light them?
- What
should you do if the stopper pops off and/or you are splashed with acid.
- After
youíre done with your matches, where should you put them BEFORE they go in
the trash can?

Questions:
- What
type of reaction is occurring in the Erlenmeyer Flask? (Synthesis,
Decomposition, Single Displacement, Double Displacement or Combustion).
- Write
out a BALANCED equation for the reaction in the Erlenmeyer flask.
(You need to know that Aluminum has an oxidation number of +3.
Hydrogen is +1. Chloride
is Ė1. The formula for
hydrochloric acid is HCl)
- What
are the reactants in this equation?
- What
are the products in this equation?
- Look
at your balanced equation (from Question #2). If you had 4 moles of Aluminum for this reaction, how
many moles of Hydrogen would you produce? (Realize that equations are
balanced in moles, so an equation simply tells you the ratio of moles of
reactant to moles of product)
- What
type of reaction is occurring in the test tube? (Synthesis, Decomposition,
Single Displacement, Double Displacement or Combustion).
- Write
out a BALANCED equation for the reaction in the test tube.
(Just inside the test tube, no aluminum or HCl is in
- What
are the reactants in this equation?
- What
are the products in the equation?
- Look
at the balanced equation (from Question #7).
If you had 2 moles of oxygen, how many moles of water would be
produced?
- Why
do you think I asked you to fill the test tube half-way with water.
Why not all the way full?
What is due for this lab?
Turn in the pre-lab questions and the questions only.
NEATNESS
COUNTS

